Antiplatelets are not as effective as anticoagulants. Warfarin, one of the most widely used anticoagulants (blood thinners) worldwide, is used to treat and prevent blood clots that can result in deep vein thrombosis (DVT), heart attacks, and strokes. Warfarin is one of the most difficult medications to properly administer because of the wide variations in each person’s metabolism, despite its effectiveness. Warfarin effectively inhibits the clotting pathway that is dependent on vitamin K. Vitamin K dependent clotting factors include 2,7,9,10 as well as protein C and S (natural anticoagulants). (PA145011113 @ Www.Pharmgkb.Org, n.d.)
Mechanism of Action
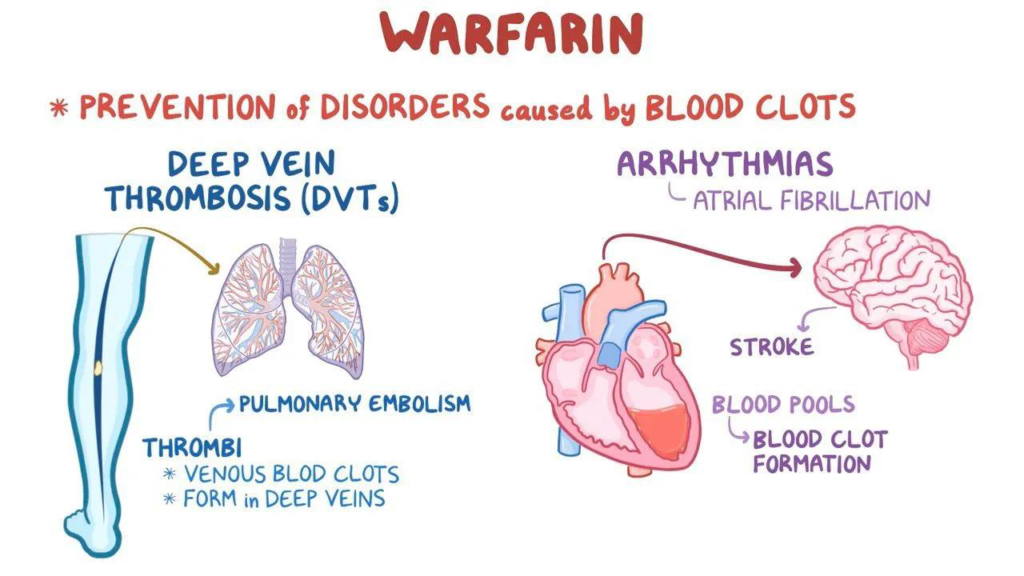
The vitamin K epoxide reductase complex subunit 1 (VKORC1), an enzyme essential to the activation of accessible vitamin K, is competitively inhibited by warfarin. By reducing functional vitamin K reserves in this way, warfarin can reduce the production of active clotting components. The liver needs vitamin K to produce coagulation regulator proteins C and S as well as
coagulation factors II, VII, IX, and X. Vitamin K is an essential cofactor for the synthesis of various clotting components that are dependent on it. (9b5dedb4c4e8b0a45b799444e4e36e177e128aab @ Www.Ncbi.Nlm.Nih.Gov, n.d.)
Goal of warfarin Therapy
By disrupting the pathway of coagulation, warfarin, an anticoagulant, reduces the incidence and intensity of thrombus formation. Patients with atrial fibrillation or deep vein thrombosis have a greater chance of developing thrombus because their blood flow is decreased. Tissue damage is the cause of this elevated coagulability in patients with heart valve disease or valve replacements. Pulmonary emboli, which stop blood flow to a section of the lung tissue, can be produced by thrombi associated with venous thrombosis as they travel to the lungs. Ischemic strokes can be brought on by thrombi that originate in the heart and go to the brain. The main objective of warfarin therapy is to prevent these occurrences. (DB00682 @ Go.Drugbank.Com, n.d.)
Metabolism of Warfarin
Two enantiomers of the anticoagulant drug warfarin are metabolized differently by human cytochrome P450 (CYP).
- R-warfarin – Carbonyl reductases transform R-warfarin into Dia stereoisomeric alcohols, while CYP1A2 breaks it down into 6- and 8-hydroxywarfarin and CYP3A4 breaks it down into 10-hydroxywarfarin. The metabolites of benzylic alcohol and 10-hydroxywarfarin undergo an elimination mechanism to produce dehydrowarfarin.
- S-warfarin – CYP2C9 is the primary enzyme that converts S-warfarin to 7-hydroxywarfarin.(S0163725896001404 @ Www.Sciencedirect.Com, n.d.)
Compared to R-warfarin, S-warfarin is three to five times more effective.
(5ad3b8ecd5d2a98e0495233f7d07c277d3e97ef1 @ Www.Ncbi.Nlm.Nih.Gov, n.d.)
Why Warfarin’s Metabolism is critical?
The metabolism of warfarin varies greatly from person to person because of dietary variables, pharmacological interactions, and genetic variations.
- Genetic Variability
Genetic differences cause certain people to metabolize Warfarin at varying rates, which can result in either under-anticoagulation (clotting risk) or over-anticoagulation (bleeding risk).
Polymorphisms in CYP2C9: People with decreased CYP2C9 activity have a slower rate of Warfarin clearance and a higher risk of bleeding.
VKORC1 mutations: Changes in this gene affect the sensitivity of Warfarin, necessitating dosage modifications.
Variations in CYP4F2: Affect vitamin K availability, which in turn affects the Warfarin response. - Drug-Drug Interactions
Even if the INR does not rise, the risk of bleeding can be raised by taking any drug that affects clotting, including selective serotonin reuptake inhibitors (SSRIs), nonsteroidal anti-inflammatory medications (NSAIDs), antiplatelets, and other anticoagulants.
Drugs that enhance Warfarin’s effect (increased bleeding risk):
o Antibiotics (e.g., Metronidazole, Trimethoprim-Sulfamethoxazole) – Inhibit Warfarin metabolism
o Antifungals (e.g., Fluconazole) – CYP2C9 inhibitor
o NSAIDs & Aspirin – Increase bleeding risk by inhibiting platelet function
o Amiodarone – Inhibits Warfarin metabolism, increasing its effect
Drugs that reduce Warfarin’s effect (increased clotting risk):
o Rifampin – Induces CYP enzymes, increasing Warfarin metabolism
o Barbiturates & Carbamazepine – Increase Warfarin breakdown
o Vitamin K supplements – Directly counteract Warfarin’s anticoagulant effect - Dietary Influence
Since Warfarin’s mechanism of action is closely tied to vitamin K, diet plays a crucial role in maintaining stable therapy.
High vitamin K foods (reduce Warfarin effect, increasing clot risk): Leafy greens like spinach, kale, and broccoli.
Low vitamin K diet (increase Warfarin effect, increasing bleeding risk): Sudden reductions in vitamin K intake can make Warfarin too potent.
Alcohol consumption: Excessive alcohol can inhibit Warfarin metabolism, increasing bleeding risk. - Liver and Kidney Function
Because Warfarin is metabolized in the liver, any liver disease, such as cirrhosis, might decrease its metabolism, increasing the risk of bleeding and drug levels. The clearance of Warfarin may also be changed in patients with renal impairment, demanding careful INR monitoring.
Adverse Effects of Warfarin
Since bleeding is the most frequent side effect, it is important to closely monitor signs and symptoms after starting warfarin.
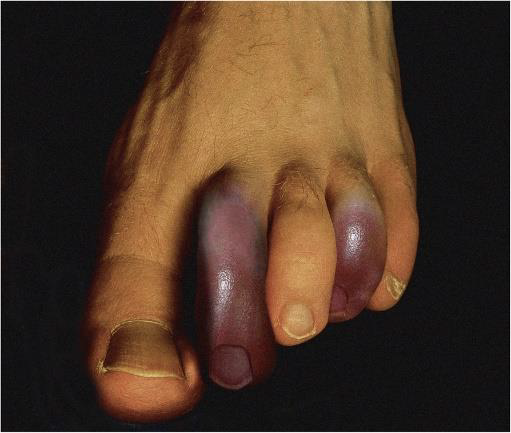
Purple/Blue toe Syndrome
When atherosclerotic plaques break in patients, thrombus usually forms. Plaque rupture in these anticoagulated patients may permit cholesterol to escape from the lipid core as cholesterol micro emboli or atheroemboli. Smaller than thrombi, these emboli obstruct vessels that are typically less
than 200 μm in diameter. Depending on where the obstruction is, there are a variety of consequences. Purple or blue toe syndrome, central nervous system ischemia, acute kidney injury or worsening of existing kidney disease, and vision problems are among the effects.
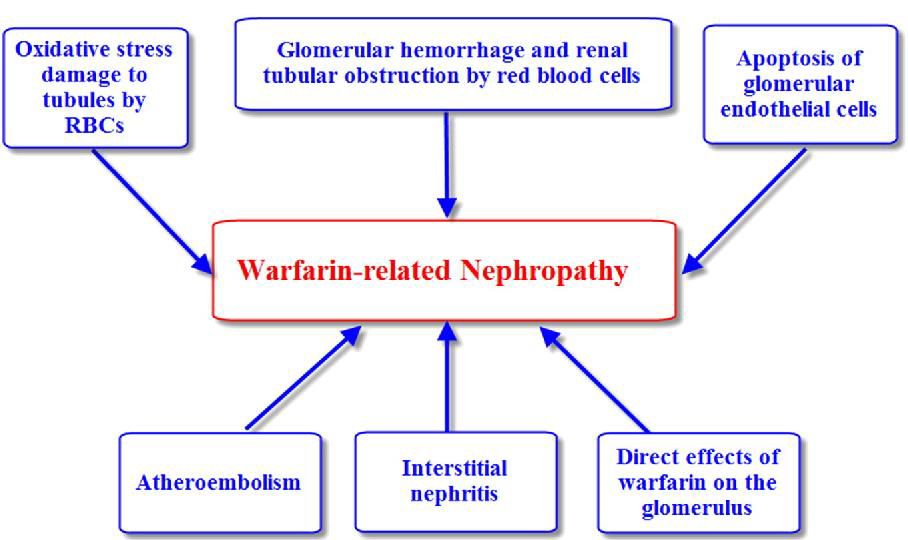
Warfarin-related nephropathy
Anticoagulation tends to be a mediating factor in warfarin-related nephropathy, an apparently spontaneous kidney injury or worsening of existing renal disease associated with warfarin therapy. In this instance, nephropathy seems to be caused by increased red blood cell passage through the glomerulus, which leads to red blood cell casts obstructing the renal tubules. Previous renal disease exacerbates this or may cause it. At INRs above 3.0, there is an increased risk of warfarin-related nephropathy; however, risk does not rise in proportion to INR after this. (DB00682 @ Go.Drugbank.Com, n.d.)
Tissue necrosis
Tissue necrosis
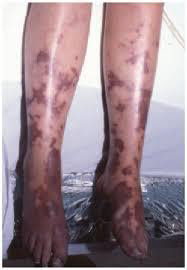
Tissue necrosis has been linked to people with deficits in proteins C or S and typically appears a few days after beginning warfarin. Proteins C and S have half-lives of eight and twenty-four hours, respectively, and are anticoagulant factors. The half-lives of thrombin (factor II), coagulation factors IX, X, VII, and others are 24, 36, 6, and 50 hours, respectively. This indicates that, aside from factor VII, proteins C and S are inactivated earlier than pro-coagulation proteins. Warfarin reduces the synthesis of both of
these naturally occurring anticoagulant proteins, which leads to a prothrombotic condition. (5a3644f0def6cd432a882d017cc22726fe4fa1f3 @ Www.Ncbi.Nlm.Nih.Gov, n.d.)
Calciphylaxis/ Increased calcium levels
In patients with or without end-stage renal disease, calciphylaxis, also referred to as calcium uremic arteriolopathy, is another rare adverse effect. Calciphylaxis is a rare and possibly deadly condition that causes calcium to build up in the small blood capillaries that surround the skin. This can lead to painful, non-healing ulcers, as well as sepsis and death. Warfarin is thought to prevent vitamin K recycling since VKA is necessary for the carboxylation of matrix Gla protein. By inhibiting the carboxylation stage of its synthesis, this protein, an anti-calcification factor, causes the calcification balance to shift in favor of calciphylaxis.
Dose of Warfarin
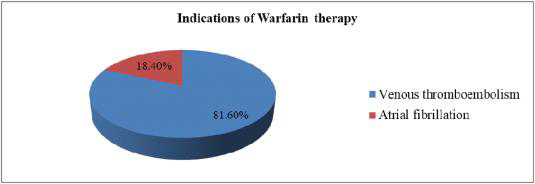
Venous Thrombosis
Treatment and prevention of pulmonary embolism (PE), venous thrombosis, and its progression
Initial dose: 2-5 mg PO/IV qDay for 2 days, OR 10 mg PO for 2 days in healthy individuals
On day 1 or 2 of LMWH or unfractionated heparin therapy, start taking warfarin. Continue taking it until the required INR is reached, then stop taking heparin.
DVT and PE treatment
On the first or second day of parenteral anticoagulant medication (such as LMWH or unfractionated heparin), start taking warfarin.
For at least five days, take warfarin and a parenteral anticoagulant together. After the required INR (>2.0) is maintained for twenty-four hours, stop the parenteral medication. (Coumadin-Jantoven-Warfarin-342182 @ Reference.Medscape.Com, n.d.)
Approaches for Treatment
To reduce risk of tissue necrosis
Concurrent heparin therapy for 5–7 days may minimize the incidence of necrosis when beginning warfarin because heparin decreases blood coagulation that happens during early warfarin treatment.
Bridging Therapy
Bridging anticoagulation is stopping warfarin and injecting a short-acting blood thinner, typically low-molecular-weight heparin, subcutaneously 10–12 days before to the procedure or surgery. After discontinuing warfarin five to six days before surgery (to allow the anticoagulant effect to wane), bridging anticoagulation is started three days before surgery, with the last dose given twenty-four hours before surgery. Warfarin is started simultaneously with the resumption of bridging, which cannot be started earlier than 24 hours following surgery. (CIRCULATIONAHA @ Www.Ahajournals.Org, n.d.)
Monitoring of Warfarin Therapy
Regular monitoring of warfarin users is necessary to guarantee the safety and effectiveness of the drug. Regular blood tests are advised to evaluate the PT and INR. The INR makes it possible to standardize the PT measurement according to the thromboplastin reagent that is being used in a lab. The time it takes for the blood to clot is known as the PT. Therefore, monitoring the INR while taking warfarin is more effective than PT because it allows for a consistent measurement that is unaffected by variations caused by various laboratory sites.
A healthy patient who Is not on anticoagulant medication has an INR of about 1.0. Consequently, it takes two or three times longer for blood to clot in a patient with an INR of 2.0 or 3.0. The INR target for the majority of warfarin patients is between two and three.
Patients must also be regularly monitored for signs of active bleeding during their treatment. Any bleeding symptoms, such as hematomas, nosebleeds, and black, tarry stools, must be closely monitored. It is important to ascertain the patient’s baseline hemoglobin and hematocrit levels prior to initiating warfarin. These levels should be checked every six months while the patient is receiving treatment.
To assist prevent potential side effects associated with supratherapeutic or subtherapeutic anticoagulation, it is necessary to monitor drug-drug, drug-herbal, drug-food, and drug-disease condition interactions. (5ad3b8ecd5d2a98e0495233f7d07c277d3e97ef1 @ Www.Ncbi.Nlm.Nih.Gov, n.d.)
Toxicity
The INR and the existence of bleeding determine the course of treatment for a patient with warfarin toxicity.
| INR more than 3 but no bleeding | Immediately stop Warfarin 1-2mg oral dose of Vitamin K Measure INR within 24 hours Resume warfarin at a lower dose when INR is within therapeutic range. |
| INR more than 10 but no bleeding | Stop warfarin 3-5mg oral dose of vitamin K Measure INR within 24 hours Resume warfarin at a lower dose when INR is within therapeutic range. |
| INR more than 3 (3.5-10) and bleeding | Immediately stop warfarin Intravenous continuous infusion of Vitamin K (5-10mg) |
If continuous infusion of vitamin K is not working/available, consider:
- Prothrombin concentrate complex 15-30 IU/kg
- Fresh frozen plasma (10 – 15 ml/kg) if prothrombin concentrate complex is not available
- Warfarin reversal – KCENTRA (48917839d17a3a8e6becdb13620dc9fa5dd7d979 @ Litfl.Com, n.d.)
Contraindication
- Warfarin is contraindicated in patients with:
- Hypersensitivity to warfarin or any component of the formulation
- Significant hemorrhage risk
- active gastrointestinal ulceration or bleeding
- central nervous system (CNS) bleeding
- Bleeding from the respiratory or genitourinary tracts
- diagnosing an aortic aneurysm
- patients having spinal or epidural punctures or other medical procedures that could cause severe bleeding
- Recent or planned eye, central nervous system, or traumatizing surgery that leaves a lot of exposed surfaces
- Risk of abortion, preeclampsia, or eclampsia;
- Bleeding related to pericarditis, pericardial effusion, or bacterial endocarditis
- Unsupervised patients with disorders linked to a high risk of medication nonadherence;
- Pregnancy, with the exception of individuals with artificial heart valves who are more susceptible to thromboembolism
- Malignant hypertension
- Major regional or lumbar block anesthesia;
Frequently Asked Questions
- What are indications to use warfarin?
One of the most used anticoagulants (blood thinners) in the world, warfarin is recommended to treat and prevent blood clots that can cause heart attacks, strokes, and deep vein thrombosis (DVT). - Is warfarin metabolized in liver?
The liver is where the majority of warfarin metabolism takes place. It involves the cytochrome P450, and in particular, the CYP2C9 isoenzyme. - Why warfarin is bridged with LMWH?
In order to reduce the amount of time that patients are not taking anticoagulants and, consequently, the risk of tissue necrosis, warfarin is bridged with LMWH. - What foods can interfere with Warfarin therapy?
By encouraging blood clotting, foods strong in vitamin K, such as leafy greens (spinach, kale, and broccoli), can lessen the effectiveness of Warfarin. Instead of completely avoiding these items, patients should continue to consume a steady amount of vitamin K. - How often should INR be monitored for Warfarin patients?
New patients: INR should be checked frequently (e.g., every 2-3 days initially).
Stable patients: INR testing is typically done every 2-4 weeks unless there are changes in diet, medications, or health conditions.
REFERENCES
- 48917839d17a3a8e6becdb13620dc9fa5dd7d979 @ litfl.com. (n.d.). https://litfl.com/warfarin-toxicity/
- 5a3644f0def6cd432a882d017cc22726fe4fa1f3 @ www.ncbi.nlm.nih.gov. (n.d.). https://www.ncbi.nlm.nih.gov/books/NBK441964/
- 5ad3b8ecd5d2a98e0495233f7d07c277d3e97ef1 @ www.ncbi.nlm.nih.gov. (n.d.). https://www.ncbi.nlm.nih.gov/books/NBK470313/
- 9b5dedb4c4e8b0a45b799444e4e36e177e128aab @ www.ncbi.nlm.nih.gov. (n.d.). https://www.ncbi.nlm.nih.gov/books/NBK470313/#:~:text=Go to%3A-,Mechanism of Action,synthesis of active clotting factors
- CIRCULATIONAHA @ www.ahajournals.org. (n.d.). https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.110.955369
- Coumadin-Jantoven-Warfarin-342182 @ Reference.Medscape.Com. (n.d.). https://reference.medscape.com/drug/coumadin-jantoven-warfarin-342182
- DB00682 @ go.drugbank.com. (n.d.). https://go.drugbank.com/drugs/DB00682
- PA145011113 @ www.pharmgkb.org. (n.d.). https://www.pharmgkb.org/pathway/PA145011113
- S0163725896001404 @ www.sciencedirect.com. (n.d.). https://www.sciencedirect.com/science/article/abs/pii/S0163725896001404