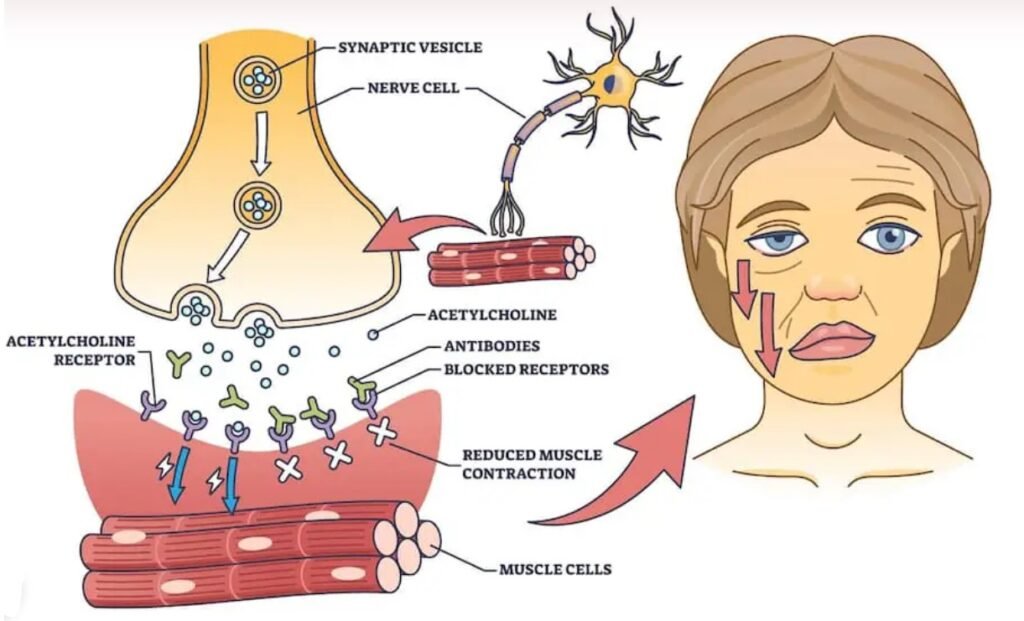
Myasthenia gravis is a chronic autoimmune disease affecting the neuromuscular junction. This leads to acutely evolving, fatigable weakness and significantly affects ocular, facial, and bulbar muscles. The most common cause of Myasthenia Gravis is the attack of antibodies to acetylcholine receptors in the post-junctional membrane of the neuromuscular junction. This results in blockage of neuromuscular transmission and impairing the communication between nerves and muscles. This antibodies attack reduces the number of acetylcholine receptors and damages the end plate. This disease is three times more common in women. This neuromuscular junction disease can immensely affect day-to-day activities, but with appropriate diagnosis and prompt treatment, patients can manage their symptoms effectively.
Understanding the Neuromuscular Junction
To understand the effect of Myasthenia gravis, it’s crucial to understand the neuromuscular junction. The neuromuscular junction is the bridge between the nervous system and the muscular system. It is the point where motor neurons transmit signals to muscle fibers, enabling the process of contraction of the skeletal muscles. This process involves:
- Nerve impulse transmission – A motor neuron releases acetylcholine (ACh) into the synaptic space.
- Receptor activation – ACh binds to receptors on the muscle fiber membrane, triggering muscle contraction.
- Enzyme activity – Acetylcholinesterase breaks down acetylcholine to terminate the signal.
In Myasthenia gravis, the immune system produces autoantibodies that block, alter, or destroy acetylcholine receptors, reducing signal transmission and leading to muscle weakness.
Etiology
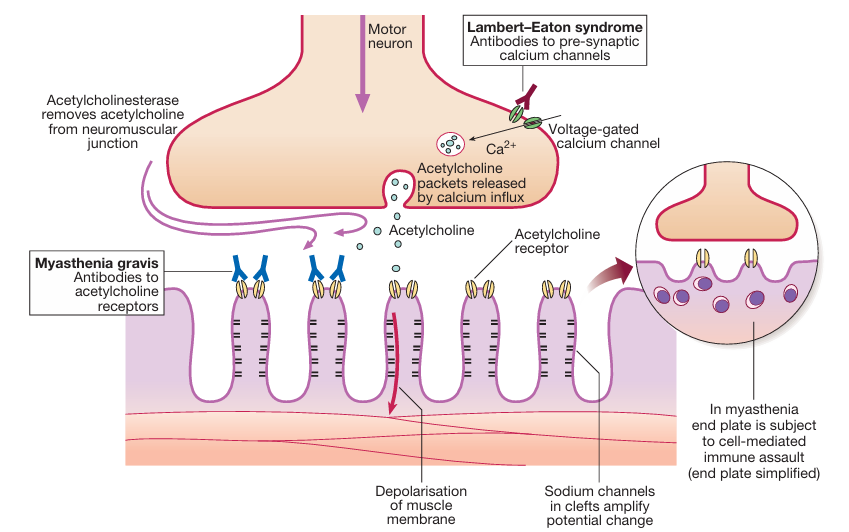
Myasthenia gravis is an autoimmune disorder, meaning the body’s immune system mistakenly attacks its tissues. The causative factor is unknown, but the disorder may have a genetic link. Some potential triggers include:
- Thymus abnormalities: The thymus gland plays an important role in immune system regulation. 15% Myasthenia gravis patients have thymic hyperplasia or tumors, which may contribute to disease development.
- Genetic Factors: Although Myasthenia gravis is not directly inherited, genetic factors may increase susceptibility.
- Environmental factors: Infections, stress, surgery, and certain medications (e.g., beta-blockers, quinidine, aminoglycosides) may exacerbate the symptoms of Myasthenia gravis.
Symptoms of Myasthenia Gravis
Myasthenia gravis symptoms can vary in severity and presentation. The most evident feature is fatigable muscle weakness that worsens with activity and improves with rest. Key symptoms include:
- 1. Ocular Symptoms: The initial symptoms are intermittent Ptosis (drooping eyelids) or Diplopia (double vision). Other ocular symptoms include weakness in eye movement muscles and resting of the eyelids (looking downwards), which may be followed by increased reflex elevation with up-gaze (the so-called Cogan’s lid twitch sign).
- 2. Bulbar Symptoms: Weakness of chewing, difficulty swallowing (dysphagia), Slurred speech (dysarthria), and facial weakness may occur.
- 3. Generalized Muscle Weakness: Weakness in arms, legs (any limb may be affected), and neck muscles, fatigueness, and difficulty holding up the head, or maintaining posture can also occur.
- 4.Respiratory Complications: Severe cases may lead to myasthenic crisis, a life-threatening condition where respiratory muscles become too weak to function properly, requiring ventilator support in abrupt onset. Respiratory failure is an avoidable cause of death.
Diagnosis of Myasthenia Gravis:
Diagnosing Myasthenia gravis is quite challenging due to its fluctuating symptoms. Its confirmatory diagnosis involves a combination of clinical evaluation and specialized tests:
- 1. History and Physical Examination: It’s crucial to take a complete history of the patient’s symptoms and medical condition and perform proper physical examination.
- 2. Tensilon Test (Edrophonium Test): This test involves the use of a medicine called tensilon which inhibits the acetylcholine breakdown and hence increases the availability of acetylcholine. It is given intravenously at a dose of 2 mg at a time to a total of 10 mg. 30 seconds after the injection, the patient is observed for improvement in muscle strength. If the muscle strength increases in response to Tensilon, then the diagnosis of myasthenia gravis is confirmed. Another drug, short-acting acetylcholinesterase inhibitor (edrophonium) may be used to temporarily improve muscle strength.
- 3. Electromyography (EMG): In EMG, electrical activity in muscles is measured, detecting impaired nerve-to-muscle transmission. EMG is considered the most sensitive test for diagnosis of myasthenia gravis as it detects impaired nerve-to-muscle transmission.
- 4. Serologic Tests: Tests are performed to detect the presence of autoantibodies against acetylcholine receptors (AChR) or muscle-specific kinase (MuSK), aiding diagnosis.
- 5. Ice Pack Test: Cooling the eyelid can temporarily improve ptosis in MG patients.
- 6. CT/MRI Scan: These can be performed to detect thymus enlargement.
- 7. Pulmonary Function Tests: This is to assess respiratory muscle function, especially in severe cases of Myasthenia gravis.
Treatment and Management
While there is no permanent cure for Myasthenia gravis, various treatments help manage symptoms and improve quality of life. The goals of pharmacological treatment for Myasthenia gravis are to maximize the activity of acetylcholine at remaining receptors in the neuromuscular junctions and to limit the immunological attack on motor end plates.
1. Acetylcholinesterase Inhibitors:
The first line treatment is Pyridostigmine (Mestinon). It is commonly prescribed to increase acetylcholine levels at the Neuromuscular Junction, temporarily improving muscle strength.
2. Immunosuppressive Therapy
- Corticosteroids (e.g., prednisone) reduce immune system activity, treatment should continue in hospital and continue for months or years which increases the chances of serious adverse effects.
- Immunosuppressants (e.g., azathioprine, mycophenolate mofetil) are used in cases requiring long-term immune modulation.
3.Plasmapheresis:
It may provide a marked improvement in the symptoms process. It is a process by which plasma is separated from formed elements of blood, plasma is discarded and RBC electrolytes are returned to the clients. This way antibodies are removed from the patient’s blood and this process is normally reserved for patients with myasthenic crises.
4. Intravenous Immunoglobulin (IVIG):
This lowers the levels of antibodies and significantly improves muscle weakness.
5. Thymectomy:
Surgical removal of the thymus gland may be required and improve symptoms, especially in patients with thymoma or generalized Myasthenia gravis.
6. Monoclonal Antibody Therapy:
Rituximab and eculizumab are newer therapies targeting specific immune components and are beneficial for refractory Myasthenia gravis cases.
Lifestyle and Coping Strategies
Management of this neuromuscular disease; Myasthenia gravis involves lifestyle adjustments to minimize symptom flare-ups and improve daily functioning. Some of the coping strategies are listed below:
- Energy Conservation: The patient should plan activities with rest periods in between to prevent extensive muscle fatigue.
- Dietary Modifications: Dietary modifications involve soft foods and small, frequent meals which can help manage swallowing difficulties.
- Eye Care: For the management of ocular symptoms; the patient should use an eye patch or prism glasses for double vision.
- Exercise: The patient should engage in mild, supervised exercise to maintain muscle strength without overexertion.
- Stress Management: Emotional and physical stressors should be avoided, as they can trigger symptoms.
Prognosis and Future Directions
The prognosis of Myasthenia Gravis varies among individuals. With early diagnosis and an efficient treatment plan, many patients can lead normal or near-normal lives. Recent advances in immunotherapy and targeted biological treatments are offering new hope for the long-term management of Myasthenia Gravis.
Conclusion
Myasthenia gravis is a chronic neuromuscular disorder that significantly affects muscle strength. This chronic and complex disease can be effectively managed with the appropriate treatment plan. Awareness, early diagnosis, and lifestyle modifications are key factors that can play a crucial role in improving patient outcomes. As research advances, immunotherapy and targeted biological treatments hold promise for enhancing the quality of life for those living with Myasthenia gravis.
FAQS:
1. What is Myasthenia Gravis?
Myasthenia gravis is a chronic autoimmune neuromuscular disorder causing muscle weakness due to impaired communication between nerves and the muscles.
2. What are the main symptoms?
The main symptoms of Myasthenia gravis includes muscle weakness, drooping eyelids, double vision, difficulty swallowing, slurred speech, and fatigue.
3. What causes Myasthenia gravis?
The immune system releases autoantibodies which attacks acetylcholine receptors at the neuromuscular junction, often linked to thymus abnormalities, genetics, or environmental triggers.
4. How is Myasthenia gravis diagnosed?
Myasthenia gravis can be diagnosed through blood tests for antibodies, electromyography (EMG), edrophonium test, ice pack test, and imaging scans.
5. What are the treatment options?
Treatment options include medications (pyridostigmine, corticosteroids, immunosuppressants), plasmapheresis, IVIG, thymectomy, and monoclonal antibodies.