To many of us, aging appears to be a superficial passage of years. But, at the cellular level, it’s a different story of science. Whenever a cell undergoes cell division, a portion of its genetic shield, the telomeres, erodes, causing the biological clock to tick underneath. At the protective ends is the enzyme, the telomerase, whose crucial balance determines how our cells age at a graceful pace. But, when this balance is disrupted, shortening of telomeres occurs at a speedy rate that pushes the cell towards quicker dysfunction and thus, the body is exposed to accelerated aging. Comprehending this hidden series of machinery not only unravels one of the intricate studies of biology but also opens a doorway to future therapies that may help redefine cellular aging.
This blog focuses on the science of how the interplay between telomeres and telomerase contributes to the aging of human cells, and explores potential interventions for future directions to prevent its acceleration.
What are telomeres?
Telomeres are specialized repetitive DNA sequences at the ends of linear chromosomes, along with associated proteins, that help maintain the integrity of the chromosomes.
What is telomerase?
Telomerase is a specialized RNA-protein DNA polymerase complex that maintains telomere length by using its own RNA as a template for adding nucleotides to the ends of chromosomes.
How do telomeres relate to aging?
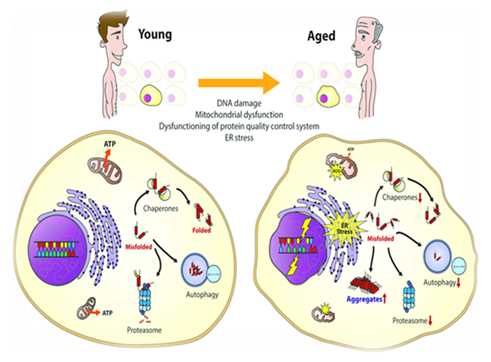
As the telomeres get shorter each time a cell copies itself, the essential DNA remains intact. Subsequently, telomeres get too short to do their job, causing our cells to age and stop functioning in the right way, and thus acting as the aging clock in every cell.

How can dysregulation of telomerase activity accelerate aging?
Telomere shortening triggers DNA Damage Response (tDDR) and cellular senescence, mimicking aging effects. While short telomeres don’t always mean a short lifespan across species, the rate of shortening and rise in short telomeres might predict lifespan.
Evidence links tDDR to aging:
· Interventions boosting health span (dietary restriction, exercise, etc.) reduce Telomere-Associated Foci (TAF).
· TAFs rise with aging and factors like inflammation, obesity.
· Telomere dysfunction drives cellular senescence via SASP, contributing to age-related tissue loss.
Moreover, the interplay of telomere-dependent and independent senescence in aging is still being explored.
Age-related Diseases Due to Cellular Senescence and Telomere Dysfunction
1. Pulmonary diseases:
· Idiopathic pulmonary fibrosis
· Chronic obstructive pulmonary disease
· Non-cystic fibrosis bronchiectasis
2. Acquired bone marrow failure syndromes:
· Aplastic Anemia
3. Metabolic diseases:
· Metabolic syndrome
· Liver Disease
· Type 2 Diabetes
4. Cardiovascular Disease:
· Cardiac Disease
· Atherosclerosis
5. Skeletal Disorders:
· Osteoarthritis
· Osteoporosis
6. Kidney Disease:
· CKD (Chronic Kidney Disease)
7. Neurodegenerative disease:
· Alzheimer’s Disease
· Parkinson’s Disease
Potential Interventions and Future Directions
Emerging research highlights the potential safety of telomerase-activating strategies to slow cellular aging. A long-term systematic review discovered that enhancement of telomerase activity in adult tissues may help stabilize telomeres and treat age-related disorders, though there was limited evidence of increased cancer risk.
Also, natural compounds, like plant extracts, have been shown to effectively boost telomerase activity in human immune cells.
These findings together suggest that regulated telomerase activation through lifestyle or dietary changes or pharmacological interventions may support healthier aging, as demonstrated in recent reviews on telomerase-mediated anti-aging interventions.
CONCLUSION
To reiterate, the interplay between telomere integrity and the activity of telomerase forms the foundation of how human cells age and function. Imbalance within this sophisticated system leads to the early emergence of cellular senescence, inflammation, and age-related diseases.
New findings may offer optimism towards targeted interventions, preserving telomere health, and slowing down the biological process of aging in the near future. Due to the advanced research, revolutionizing the realm of these molecular mechanisms, the probability of aging more gracefully becomes increasingly real as we can’t pause the clock, but we can surely learn to care for the mechanism that keeps it ticking.
Frequently Asked Questions (FAQ)
1. What is cellular aging?
Cellular aging is the progressive decline in the resistance to stress and other cellular damage, causing a gradual loss of cellular functions and resulting eventually in cell death. It is caused by genetic abnormalities and the accumulation of cellular and molecular damage due to the effects of exposure to exogenous influences.
2. Can telomerase activation completely stop aging? No, telomerase activation may slow some aspects of cellular aging. But aging depends on multiple factors, including DNA damage, metabolism, and the environment.
- Does short telomere length assure age-related diseases? No, not always. This is because short telomeres increase the risk by reducing the ability of cells to divide and repair tissues, but the development of disease also depends on many other factors, like lifestyle, genetics, and environment.
- Is telomerase active in all cells? No, because in humans, telomerase is usually active in germ cells, stem cells, and some immune cells. But almost all adult somatic cells have low or no telomerase activity at all.
- Could increasing telomerase activity raise cancer risk? Possibly, yes. While telomerase can help cells live longer, increased activity can cause uncontrolled cell division, therefore leading to cancer. Thus, regulation is crucial.
- Are there any natural ways to support healthy telomeres? Some studies suggest certain natural compounds, e.g., plant extracts, may enhance telomerase activity and support telomere maintenance. Then again, evidence isn’t clear, and more research is needed before recommending them widely.
References:
1. Rossiello, F., Jurk, D., Passos, J. F., & Di Fagagna, F. D. (2022). Telomere dysfunction in ageing and age-related diseases. Nature Cell Biology, 24(2), 135–147. https://doi.org/10.1038/s41556-022-00842-x
2. Hornsby, P. J. (2007). Telomerase and the aging process. Experimental Gerontology, 42(7), 575–581. https://doi.org/10.1016/j.exger.2007.03.007
3. Dunn, P. L., Logeswaran, D., & Chen, J. J. (2024). Telomerase-Mediated Anti-Ageing Interventions. Sub-cellular Biochemistry/Subcellular Biochemistry, 107, 1–20. https://doi.org/10.1007/978-3-031-66768-8_1