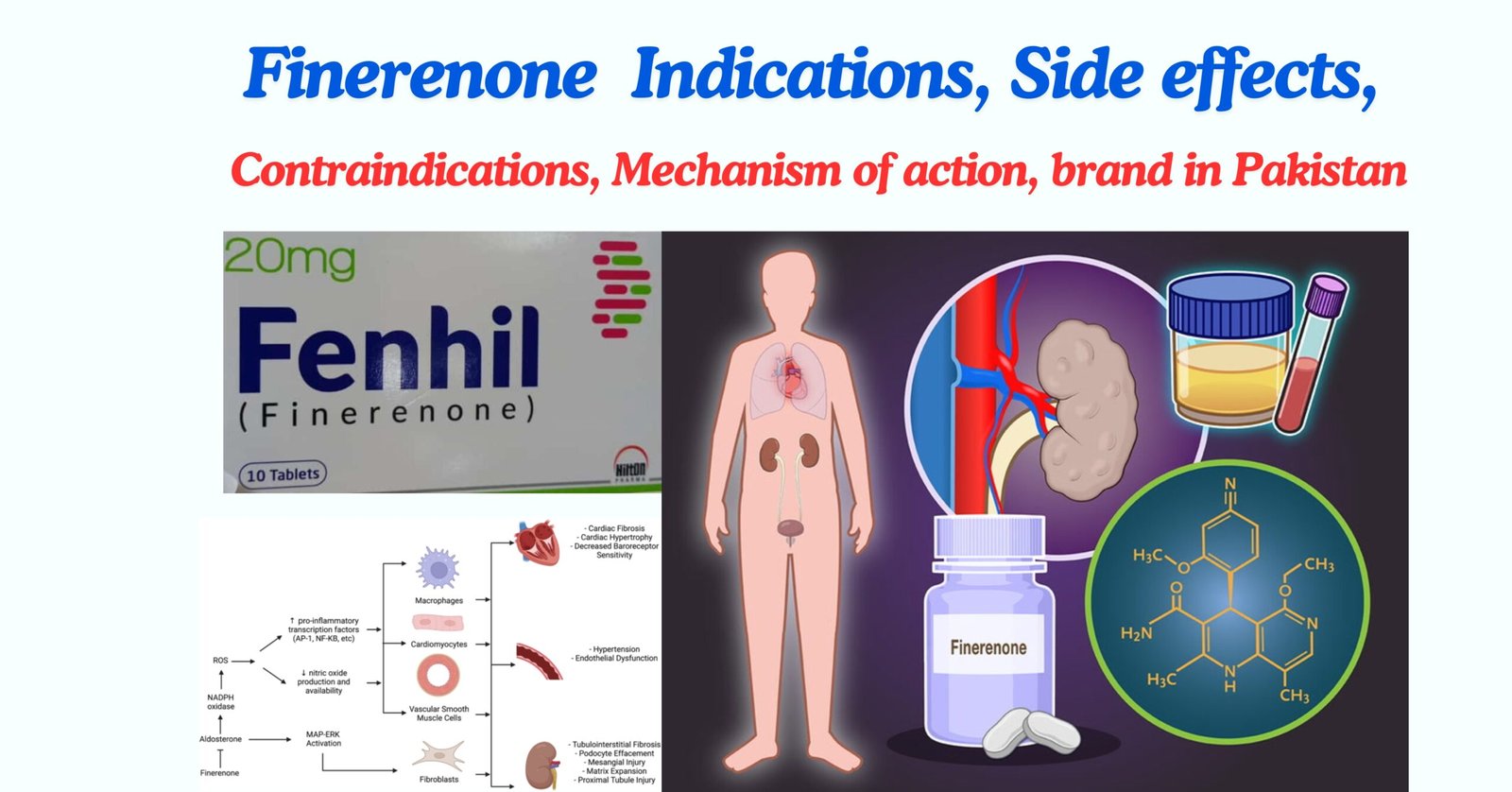
Finerenone mechanism of action
Finerenone is a non-steroidal selective antagonist of the mineralocorticoid receptor (MR) activated by aldosterone and cortisol that regulates gene transcription. Its binding to the MR gives rise to a specific receptor-ligand complex that blocks the recruitment of transcriptional coactivators involved in the expression of proinflammatory and profibrotic mediators.
Finerenone Therapeutic indications
Treatment of chronic kidney disease (stages 3 and 4 with albuminuria) associated with type 2 diabetes in adults.
Finerenone Watch Complete Video for better understanding
Finerenone administration mode
Oral route. Take with a glass of water and with or without food, they should not be taken with grapefruit or grapefruit juice. For those who cannot swallow the tablets, they may be crushed and mixed with water or soft foods, such as apple sauce, directly before oral administration.

Finerenone Contraindications
Hypersensitivity to finerenone. Concomitant use with strong CYP3A4 inhibitors e.g. itraconazole, ketoconazole, ritonavir, nelfinavir, cobicistat, clarithromycin, telithromycin, nefazodone. Addison’s disease.
Finerenone Warnings and precautions
IR, risk of hyperkalemia increases with decreased renal function.
Moderate HI, monitor and severe HI do not start treatment.
Heart failure, no studies.
Concomitant use with: moderate and weak inhibitors of CYP3A4, monitor serum potassium.
Do not use during pregnancy. Assess risk/benefit.
Finerenone Liver Failure
Severe IH, do not start treatment. Moderate IH, no initial adjustment is required. Perform additional monitoring of serum potassium and adapt such monitoring according to patient characteristics.
Finerenone Renal insufficiency
In patients with an eGFR <25 mL/min/1.73 m2
, treatment should not be initiated due to limited clinical data. In patients with an eGFR ≥15 mL/min/1.73 m2
, treatment may be continued with dose adjustment based on serum potassium. The eGFR should be measured 4 weeks after initiation to establish whether the starting dose can be increased to the recommended daily dose of 20 mg.
Finerenone Interactions
Plasma concentration increased with: strong CYP3A4 inhibitors such as itraconazole, clarithromycin, ketoconazole, ritonavir, nelfinavir, cobicistat, telithromycin or nefazodone, grapefruit juice. Contraindicated combination.
Plasma concentration decreased with: rifampicin and other strong CYP3A4 inducers (e.g. carbamazepine, phenytoin, phenobarbital, St John’s wort) or with efavirenz and other moderate CYP3A4 inducers. Combination not recommended.
Risk of hyperkalemia with: potassium-sparing diuretics (e.g. amiloride, triamterene) and other MRAs (e.g. eplerenone, esazerenone, spironolactone, canrenone), potassium supplements and trimethoprim, or trimethoprim/sulfamethoxazole.
Risk of hypotension with: other antihypertensives.
Finerenone Pregnancy
There are no data from the use of finerenone in pregnant women. Animal studies have shown reproductive toxicity. Finerenone should not be used during pregnancy unless the clinical condition of the woman requires treatment with finerenone. If a woman becomes pregnant while taking finerenone, she should be informed of the potential hazard to the fetus.
Finerenone Breastfeeding
Available pharmacokinetic/toxicological data in animals show that finerenone and its metabolites are excreted in milk. It is unknown whether finerenone/metabolites are excreted in human milk. A decision must be made whether to discontinue breast-feeding or to discontinue therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Finerenone Effects on ability to drive
Finerenone has no influence on the ability to drive and use machines.
Finerenone Adverse reactions
Hyperkalemia, hyponatremia; hypotension; pruritus; decreased glomerular filtration rate.
Finerenone Available Brands in Pakistan

- Fenhil 10 mg ( Hilton Pharma)
- Fenhil 20 mg ( Hilton Pharma)
- Fenone 10mg ( Getz Pharma )
- Fenone 20mg ( Getz Pharma )