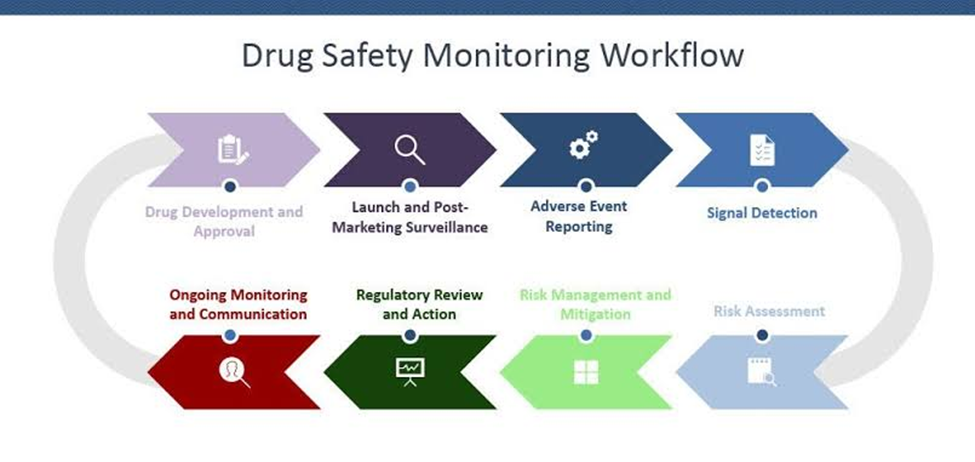
The regulatory approval of a new pharmaceutical signifies a significant accomplishment; nonetheless, it merely marks the commencement of the process. For drug safety professionals, the substantive work commences in the realm of Pharmacovigilance (PV).
Pharmacovigilance encompasses the scientific and operational tasks associated with the identification, evaluation, comprehension, and prevention of adverse effects or other drug-related issues.
Latin phrase Primum non nocere, or “first, do no harm,” is the main tenet of any drug safety system in the highly drug-dependent area of modern medicine.
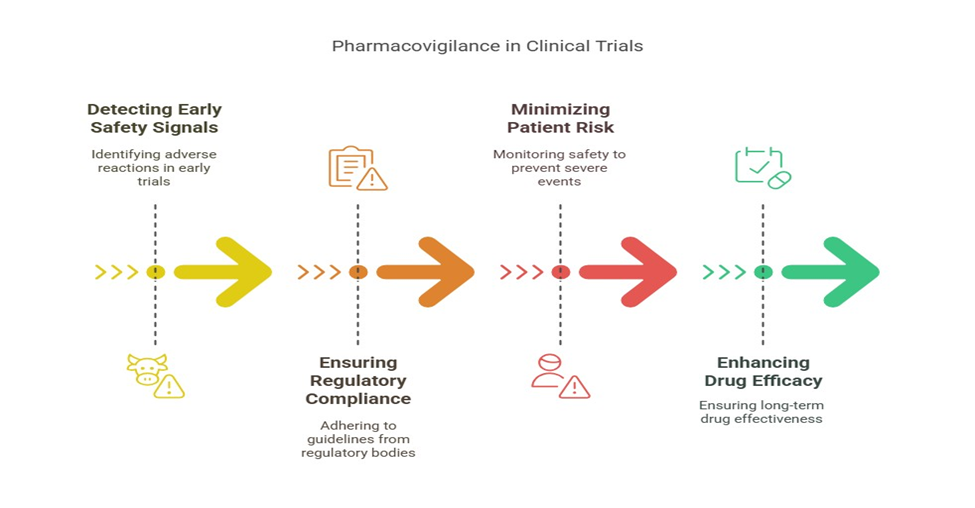
Clinical trials encompass a specific cohort of patients within regulated parameters. Upon a drug’s launch, it is utilized by millions of varied individuals, potentially uncovering hazards that were too seldom or nuanced to identify initially. Pharmacovigilance functions as the mechanism that identifies these infrequent occurrences.
Objectives
The principal objective of pharmacovigilance is to safeguard public health by identifying infrequent or prolonged side effects, recognizing novel medication interactions, comprehending hazards in particular demographics (such as pregnant women or children), and efficiently controlling and conveying these risks.
A strong reporting system, including impromptu reporting from medical personnel, patients, and caregivers, is the cornerstone of successful pharmacovigilance. In the US, organizations such as the FDA employ FAERS (FDA Adverse Event Reporting System). Drugs frequently go through additional research (Phase IV) even after approval to search for long-term safety signals. In order to search for trends that might point to a safety concern, modern PV also employs data mining and electronic health records (EHRs) to examine enormous volumes of patient data. A signal is information that suggests a possible link between a drug and an adverse occurrence, but the details of the connection are either unclear or the information is insufficient.
Reports are just the starting point. The serious analysis work takes place while the data is being processed. PV scientists apply statistical tools to test the expected historical rate in the general population against the incidence rate of a harmful event among patients taking the medicine If a signal is detected, careful data analysis is performed by the PV team, taking into consideration various parameters of the case, such as whether the signals stopped once the medicine was discontinued, and reviewing the clinical literature. Assessments are made by the experts on the probability of the medicine causing the adverse event. If evidence exists for a new, clinically important risk, production and regulatory procedures act accordingly. This could involve modifying medication labels, directly contacting physicians, executing REMS, or potentially removing the drug from distribution if required.
Pharmacovigilance is an evolving, ethical obligation that includes patients, healthcare systems, and pharmaceutical developers. Each report contributes an essential piece of information to maintain a constant evaluation of medications regarding their advantages versus risks. The next time you learn of a drug safety update, keep in mind the pharmacovigilance teams diligently working behind the scenes to verify that your prescription is safe.
” It’s pivotal that pharmacovigilance remains a precedence and that we maintain open, transparent, and formative conversations regarding the safety of drugs.”
Conclusion
In order to guarantee the safety of pharmaceutical goods during their whole lifecycle, especially after regulatory approval, pharmacovigilance is an important, ongoing, and proactive system. In conclusion, post-approval drug safety monitoring is essential because Finding Uncommon Adverse Events Ongoing Risk Management, Knowledgeable Action, and Public Health Safety In the end, strong pharmacovigilance turns drug use into an ongoing, evidence-based clinical practice rather than a post-marketing experiment.
Frequently Asked Questions (FAQs)
Q. 1 What is pharmacovigilance and why is it important?
· Pharmacovigilance is the science and practice of identifying, evaluating, comprehending, and preventing side effects or any other issue pertaining to medications or vaccines. Pharmacovigilance is crucial because it protects public health by continuously assessing the safety of medications and vaccines once they are put on the market.
Q. 2 What is pharmacovigilance intended to accomplish?
· The goal of pharmacovigilance is to identify, evaluate, comprehend, and avoid side effects or any other drug-related issues.
Q. 3 Pharmacovigilance applies to which medications?
· All pharmaceuticals, including over-the-counter and prescription medications as well as vaccines, are subject to pharmacovigilance during their whole lifecycle.
Q. 4 Pharmacovigilance involves who?
· Patients, medical experts, pharmaceutical corporations, and international regulatory bodies such as the FDA and EMA are all involved in pharmacovigilance.
Q. 5 How does one define active and passive pharmacovigilance?
· Rather than depending solely on unsolicited reports, active pharmacovigilance is the methodical, proactive gathering of data regarding drug safety. It entails targeted research and structured initiatives intended to actively gather information on adverse drug reactions (ADRs) from a specific demographic.
· Pharmacovigilance is passive. Other than encouraging medical professionals and others to report safety issues they come across while delivering clinical care, passive pharmacovigilance does not take any proactive steps to search for side effects.
Q. 6 What distinguishes passive surveillance from active surveillance?
· While active surveillance uses a system that actively and methodically looks for cases, surveillance depends on voluntary reporting from sources like hospitals and clinics. Compared to active surveillance, passive surveillance has lower costs and relies on routine procedures in collecting data, while the former is more precise and timelier, but it requires more resources.
Q. 7 What Part Does Al Play in Pharmacovigilance?
· AI systems collect information from the adverse drug event form and evaluate case validity without the need for human intervention. Improvement in the precision of diagnosis, simplification of medical processes, and optimization of treatment plans